How MRCM has adapted to changes during COVID-19 & how we test for possible fungal superinfections
One of the hottest topics in medical mycology this year has been how often COVID-19 is associated with fungal superinfections, and how best to use specialist diagnostics to distinguish between genuine infections and colonisation. This is particularly important for infections such as invasive pulmonary aspergillosis (IPA) where prompt treatment with antifungals can make the difference between life and death, but the medications involved (e.g. voriconazole) often have serious drug interactions or side effects.
MRCM have received an MFT Division of Laboratory Medicine excellence award for the way they handled the exponential increase in Aspergillus and Candida biomarker assays during the first COVID-19 wave

How has MRCM adapted during COVID-19?
We supported the critical care units not only at MFT but across the North West by testing patient samples for signs of serious fungal infections. It was challenging at times because our workload increased exponentially as the pandemic progressed, while we coped with staff needing to self-isolate or shield.
Here are some examples of how we achieved that:
- Validated safety protocols for performing our assays on respiratory samples from COVID-19 patients
- Doubled the number of fungal biomarker test performed almost overnight. Even by July, our biomarker workload was still more than 50% increased from pre-covid days!
- Teamed up with the University of Manchester’s rapid response group to help study the lung mycobiomes of COVID-19 patients
- Increased staff training for relevant procedures
- Took on all testing of superficial fungal samples (particularly nails) from GPs, going from ~5 per day to >20 per day





How common is COVID-associated aspergillosis (CAPA)?
Studies from most countries put the rate of COVID-associated aspergillosis at a similar or lower rate as influenza-associated aspergillosis. However, reported rates vary greatly because experts have yet to reach a consensus over separating colonisation from true infection.
We are still in the process of analysing our data but initial looks like there were very few cases of CAPA in this region. The UK National Mycology Reference Laboratory in Bristol (Borman et al, 2020) tested 1267 samples from 719 and found rates of probable (5%) and proven (15%) CAPA.





What do clinicians needs to know about diagnosing CAPA?
Patients at particular risk of CAPA are those with pre-existing lung disease (COPD, TB) or who have been treated with high-dose steroids or who have been on a ventilator for longer periods (>10 days).
- Read more in a blog post by LIFE Worldwide
Antigen biomarker tests (galactomannan, BDG) are much more accurate when performed on BAL fluid than serum, but performing a bronchoscopy on a COVID-19 patient risks aerosolising the virus. Suveer Singh (@Respitudoc) has made a video demonstrating ways to make this process safer: bit.ly/3e2s2WK
Galactomannan testing is not validated for other types of respiratory sample (sputum, tracheal aspirates) and these are more likely to be affected by colonisation as Aspergillus is a normal part of the mouth flora.
Watch the ECMM’s webinar about the biology, definition(s), diagnosis and management of CAPA:





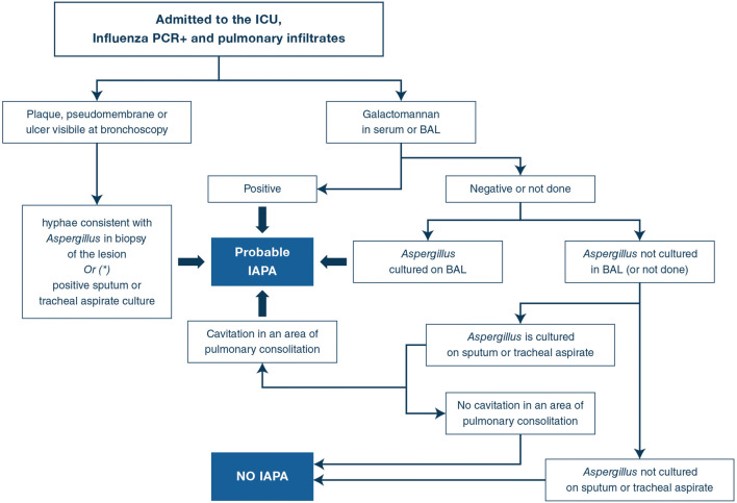
Is CAPA similar to influenza-associated pulmonary aspergillosis (IAPA)?
While we wait for more data to arrive about CAPA, many clinicians have been using what we know about IAPA for guidance.
Severe influenza is an independent risk factor for invasive aspergillosis, with high mortality rates. The AspICU algorithm (Blot et al, 2011) was designed specifically for use in critical care units, to take into account that many patients in this setting do not have the classical risk factors and clinical presentation that the EORTC/MSGERC IPA guidelines are based on (which was mainly aimed at immunocompromised patients). However, patients with IAPA are distinct from both and require their own case definition.
- Read more in a consensus statement in Intensive Care Med (Verweij et al, June 2020)