How we use pyrosequencing to detect azole resistance genes in Aspergillus fumigatus
- Pyrosequencing in practice in Journal of Antimicrobial Chemotherapy: Novak-Frazer et al (2020) Deciphering Aspergillus fumigatus cyp51A-mediated triazole resistance by pyrosequencing of respiratory specimens
- Read about the concept in Journal of Fungi: van der Torre et al (2020) Detecting Azole-Antifungal Resistance in Aspergillus fumigatus by Pyrosequencing
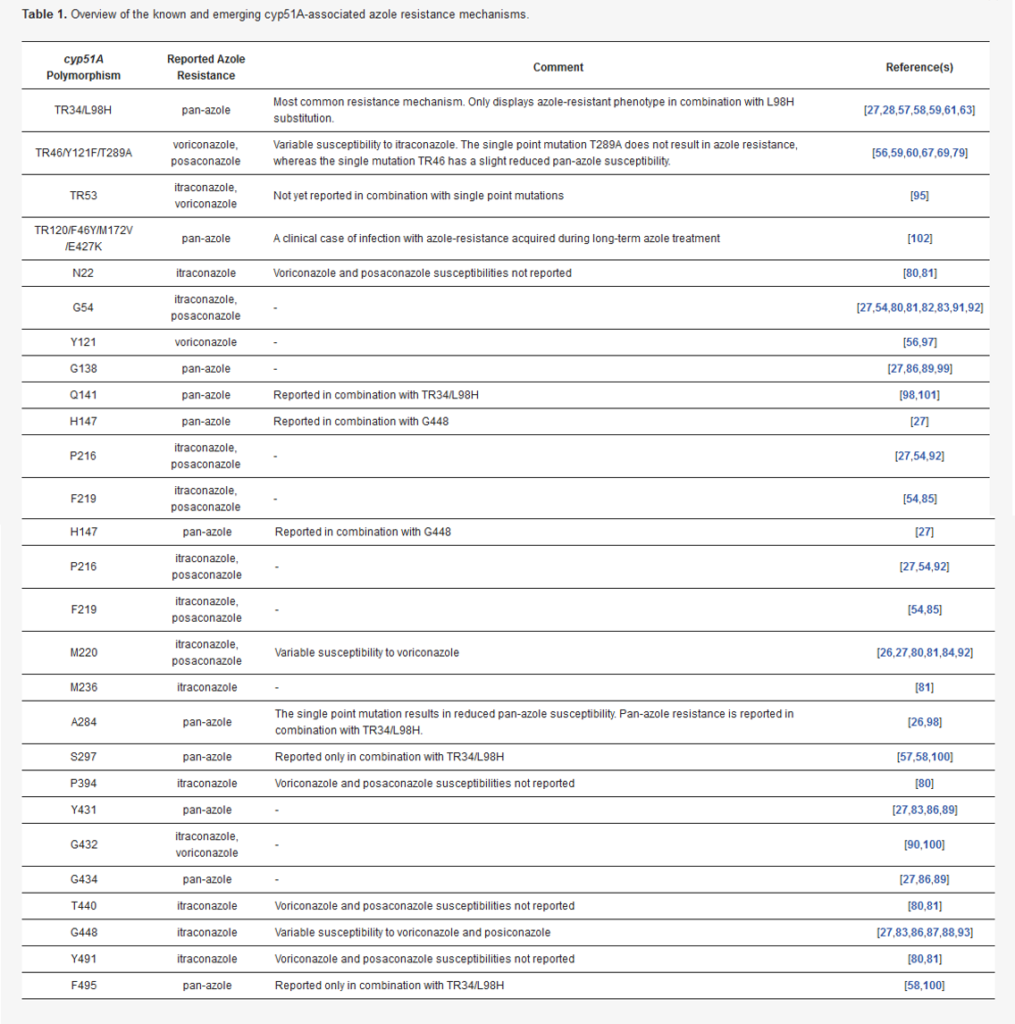
Susceptibility testing can substantially improve mortality rates from serious fungal infections such as invasive aspergillosis (IA), and this is likely to become more important as antifungal resistance rates continue to rise. However, many settings do not order this testing routinely.
Azole resistance is also a problem for patients with chronic pulmonary aspergillosis (CPA) as resistance to first-line treatments (itraconazole/voriconazole) may develop in as little as 4 months.
Conventional susceptibility testing methods (e.g. microdilution) are performed on cultures grown from patient samples on specialist agar media, but in more than half of cases the broncheolar lavage (BAL) samples are culture negative. Therefore, we developed an in-house pyrosequencing method that detects cyp51 azole-resistance alleles directly from respiratory samples.
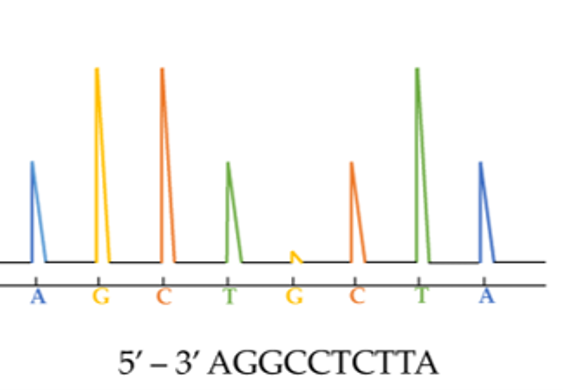
The technical bit: cyp51 gene sequences are amplified by PCR and purified using biotinylated primers. Pyrosequencing produces 150-bp reads that are compared against a reference genome.
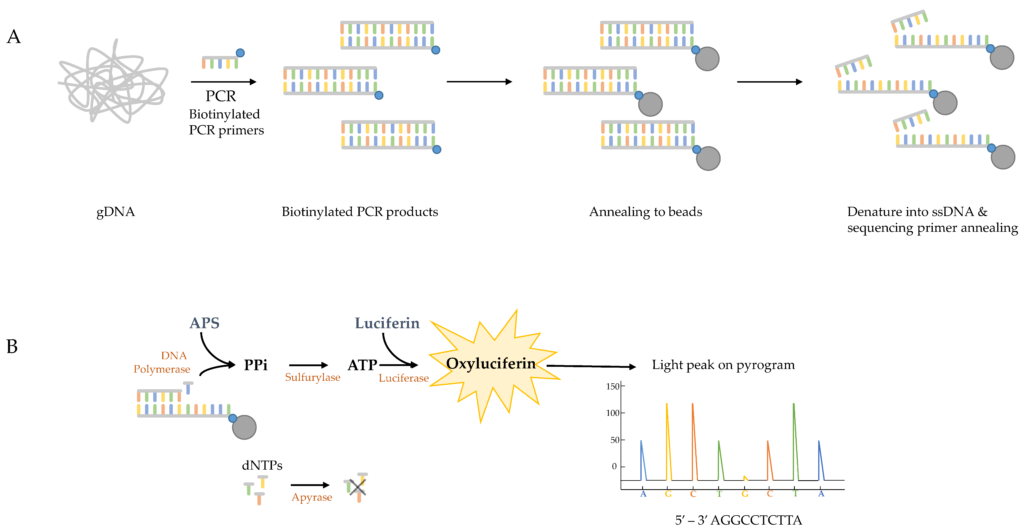
Our test has a faster turnaround time (14h process) than culture-based methods and covers a wider range of mutations than PCR-based methods . It has a similar limit of detection to existing RT-PCR assays and it can be used on samples where several different strains of fungi are present.
While we currently offer the test for Aspergillus fumigatus only, the method could be adapted for other fungi and genes of interest.