Importance of GM-BAL screening for invasive pulmonary aspergillosis in liver disease patients
Invasive pulmonary aspergillosis (IPA) is a well-known risk for severely immunocompromised patients; in recent years, however, the infection has been increasingly recognised as an emerging disease in non-neutropenic, critically ill patients. Diagnosis can be problematic in this cohort as many of the signs and symptoms of IPA are non-specific. Mortality rates remain as high as 90-100%.
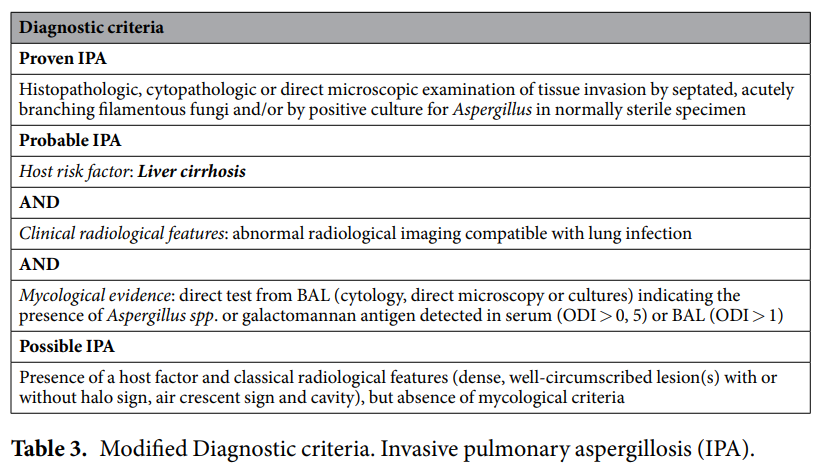
In a recent study, Tobias Lahmer and colleagues analysed the prevalence and outcome of IPA in critically ill patients with underlying liver cirrhosis. In a total cohort of 84 patients, positive galactomannan (GM) tests from bronchoalveolar lavage (BAL) samples were used to identify 12 cases of probable IPA (14%).
Key points to note:
- Scores for the Acute Physiology And Chronic Health Evaluation (APACHE II), Sequential Organ Failure Assessment (SOFA) and Model of End-Stage Liver Disease (MELD) were all higher in probable IPA cases
- Probable IPA patients needed more renal replacement therapy and more broad spectrum antibiotics
- Length of ICU stay was significantly longer in probable IPA patients (16 versus 10 days) and the mortality rate of this group was significantly higher (100% versus 65%)
- Mean intensive care unit (ICU) stay until diagnosis of IPA was 6±4 days
- An overall sensitivity of 90% and specificity of 85% was found for the GM-BAL in IPA
In conclusion, these findings demonstrate that IPA is not rare in critically ill, non-neutropenic, liver cirrhosis patients. Clinical and radiological symptoms are often non-specific in this cohort, therefore GM screening is very useful to diagnose IPA; according to the ESCMID–ECMM-ERS guidelines, GM from BAL samples has been found to be more advantageous than GM from blood samples in non-neutropenic patients.
