New CrAG lateral flow assay from Liming Bio has high sensitivity (98%) but poor PPV (50%)
CrAG screening is recommended by the WHO for HIV+ patients with a CD4 count <100 cells/μl, in order to reduce mortality from cryptococcal meningitis. Dr Edward Mpoza and colleagues in Uganda found that a New CrAG lateral flow assay from Liming Bio (China) has high sensitivity (98%) but poor specificity (90%) and positive predictive value (50%) among HIV+ patients in Kampala.
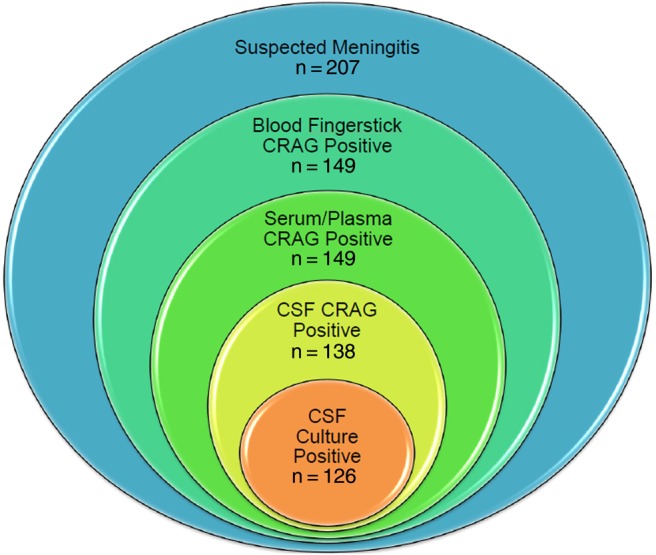
Cryptococcal meningitis accounts for 15-20% of AIDS-attributable mortality in Sub-Saharan Africa. The WHO recommends CrAG screening for all HIV+ individuals with a CD4 count <100 cells/μl, followed by pre-emptive fluconazole treatment in the case of a positive result. Point-of-care diagnostics make this easier to achieve in practice in resource-limited settings.
Several commercial lateral flow assays (LFAs) are currently available for CrAG testing but it can be tricky to compare them directly because the patient populations and experimental designs used to calculate sensitivity/specificity/PPV/NPV can vary considerably. In particular, the background (asymptomatic) rate of cryptococcal antigenamia can vary between populations, which affects the after-adjustment PPV. Useful general information can be found in the WHO guide to selecting and implementing in vitro diagnostic tests.
Dr Edward Mpoza and colleagues in Kampala (Uganda) recently published an evaluation of the new StrongStep kit from Liming Bio (China). StrongStep had a sensitivity of 98% and was able to detect antigen at dilutions down to 1:1280, while the IMMY LFA only detected down to dilutions of around 1:160. However, the specificity of StrongStep was poor (90%), resulting in a positive predictive value of only 50% after adjusting for the higher-than-average background cryptococcal antigenaemia rate in Kampala (9% versus 6% worldwide, among HIV+ patients. The authors question whether this is adequate for a national screening programme, given that an influx of false positive results would add pressure to the already-strained Ugandan healthcare system.
Please note that a positive culture from CSF is required for a definitive diagnosis of cryptococcal meningitis.

